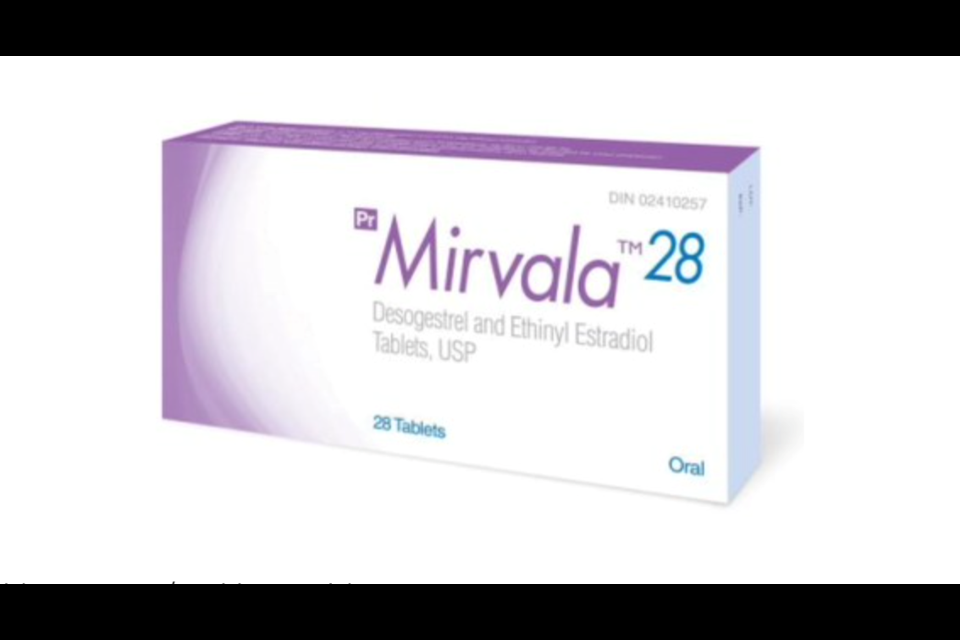
A brand of birth control pills has been recalled by Health Canada because their blister pack may contain a green placebo pill in place of an active white birth control pill.
Health Canada announced the recall of Mirvala 28 packages that contain 21 white pills on Thursday.
The packs typically have 21 active pills with hormones, and seven green placebo pills without hormones.
Taking the pills in the proper order, according to the instructions that accompany the product, is important for preventing pregnancy.
Missing an active pill or taking a placebo in place of one could result in an increased risk of pregnancy because the pill does not contain the needed active hormone.
It may also cause side effects including spotting and irregular bleeding.
The affected products are produced by Apotex Inc. with the DIN number 02410257 and the expiry date of 08/2022.
If your package has a placebo pill in place of a white pill, Health Canada says to return it to your pharmacy as soon as possible for a replacement.
If uncertain, consult your pharmacist.
If you cannot get to a pharmacy right away, users are told to take the next white pill in the package that you are due to take and contact your pharmacist for a replacement as soon as possible.
Do not miss an active pill or take a placebo in place of an active pill.
If you have missed an active pill, have no more active pills and are due to take one, or if you are unsure, use a non-hormonal effective method of birth control (like condoms) and contact your pharmacist as soon as possible.
Packages that have the correct pills in the right order do not need to be returned.