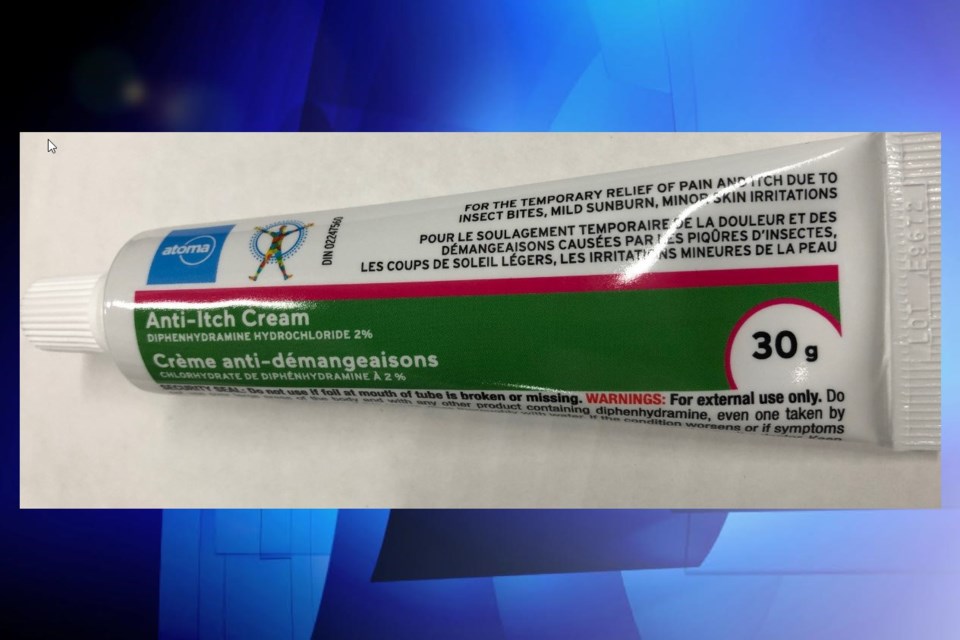
The wrong instructions on the label of one anti-itch cream for use on children has led to a recall of the product.
Health Canada says the outside text on the tube of Atoma-brand Diphenhydramine Hydrochloride 2% Anti-Itch Cream indicated the cream could be used on children under the age of two if 'supervised by an adult.'
However, they say the instructions were supposed to tell parents to 'consult a doctor' before using children of that age.
According to Health Canada, the product is a non-prescription antihistamine drug which can pose risks of irritability, agitation, restlessness, muscle spasms, seizures or trouble breathing for children under two years-old.
Makers of the anti-itch cream, Taro Pharmaceuticals Inc., alerted Health Canada about the issue on December 23rd.
Currently, Health Canada is not asking consumers to return the product, but Taro Pharmaceuticals Inc. is collecting all recalled items from stores.
The product has been sold in retail stores across Canada since July 29, 2019.
For more information, go to healthycanadians.ca.